*Based on results from randomized, placebo-controlled pilot study (Preprint: Hawkins et al., 2024).
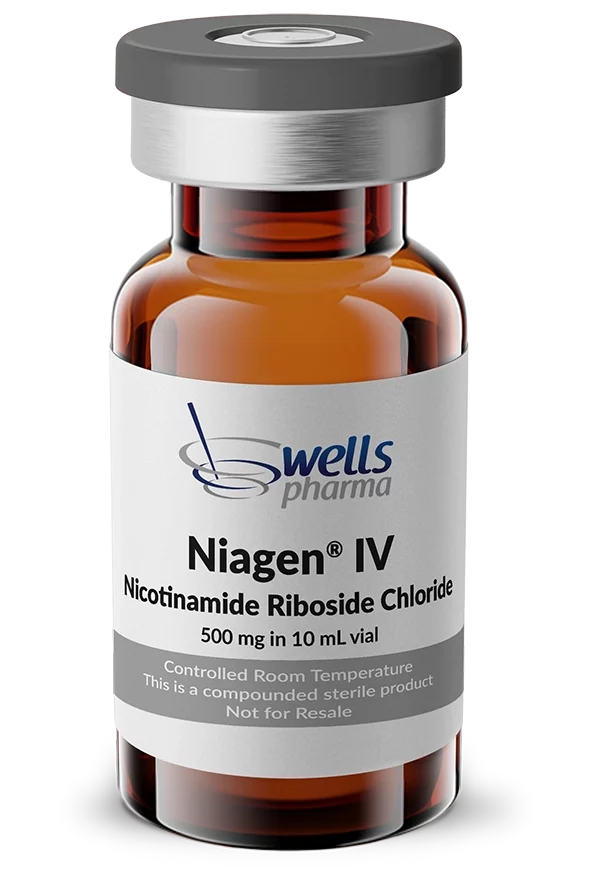
Niagen IV is a patented form of nicotinamide riboside chloride, the most effective and efficient precursor to NAD+ (nicotinamide adenine dinucleotide). Now available from Wells Pharma.
Step 1: Fill out the form online.
Step 2: Have questions? Email us at NiagenSupport@wellspharmatx.com
Let us be your compounding pharmacy of choice
Ask your physician about Wells Pharmacy Network today for all of your compounding needs. If we haven’t yet made their acquaintance, let us know and we’ll be happy to have one of our Customized Medication Specialists contact them to discuss how our commitment to superior science and service makes us a leading compounding pharmacy capable of preparing custom medications that meet their requirements.
Patients with questions about an order, prescription, or medication, please call (866) 506-2174 and ask to speak to a pharmacist.
Contact Us
Main Phone: (866) 506-2174
Prescription Fax Line: (352) 401-5650
Leave Us a Message